SAS Clinical Acceleration Repository
Support data integrity and collaboration while streamlining the path to regulatory submission.
Key Features
Help ensure data integrity and work in a validated environment while enabling collaboration and expediting regulatory submissions.
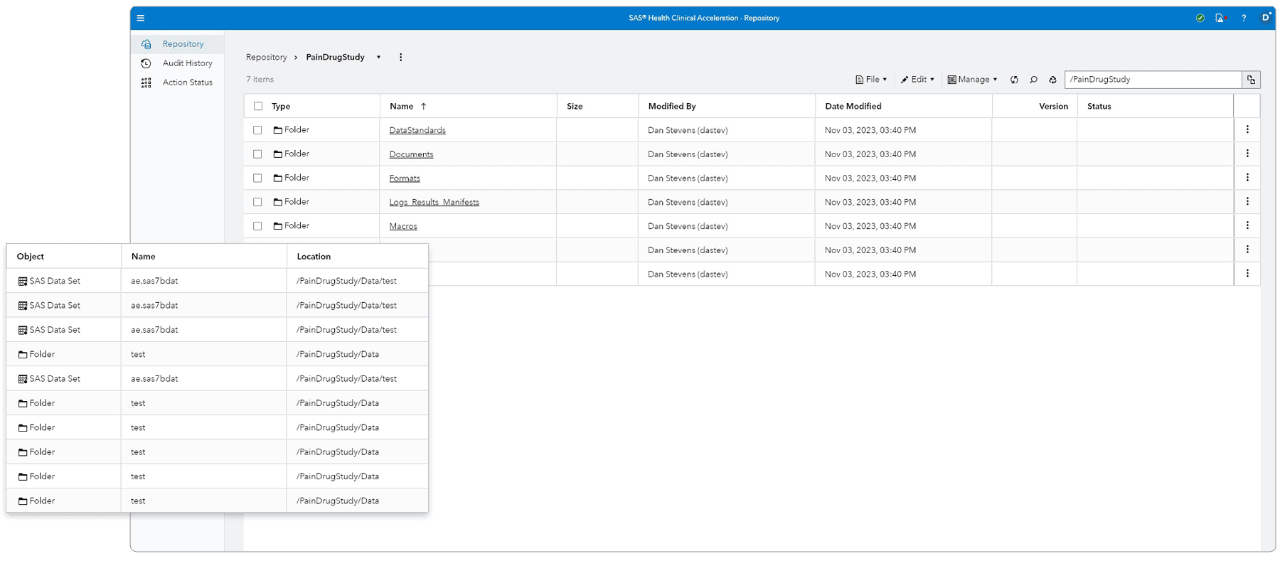
Centralized global repository
Consolidates clinical information into a single, secure, centralized global repository.
Data tracing
Traces data pedigree back to source data.
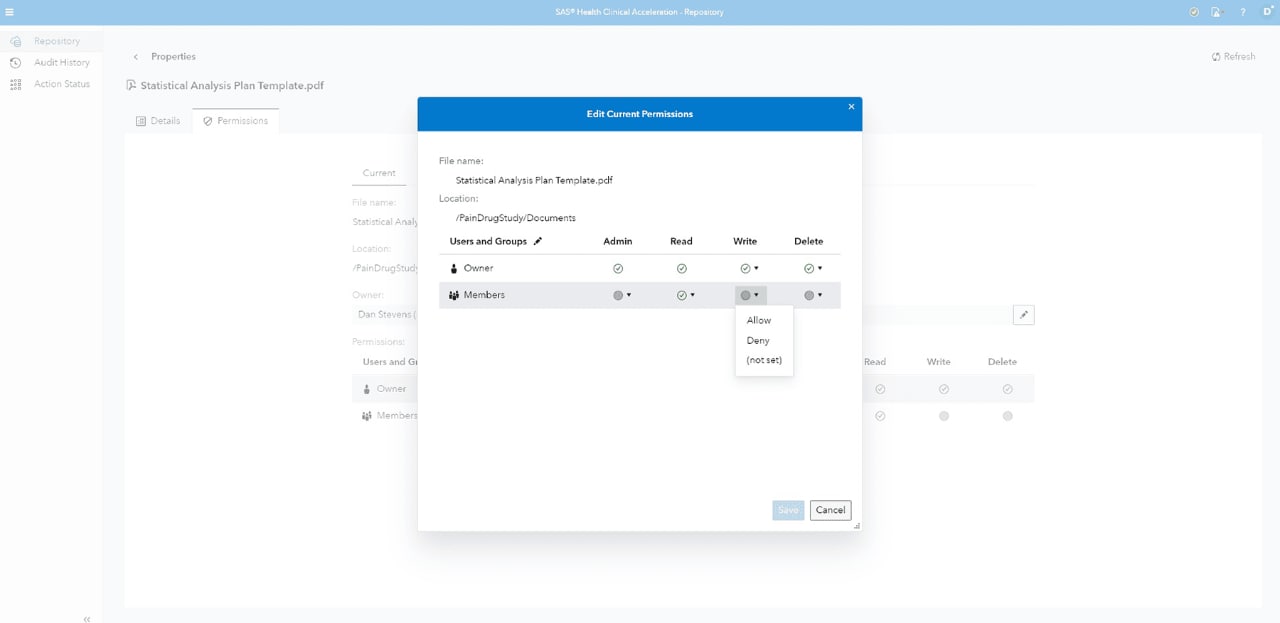
Data security
Defines data check-in/out process, audit trails, electronic signatures, versioning and role-based privileges.
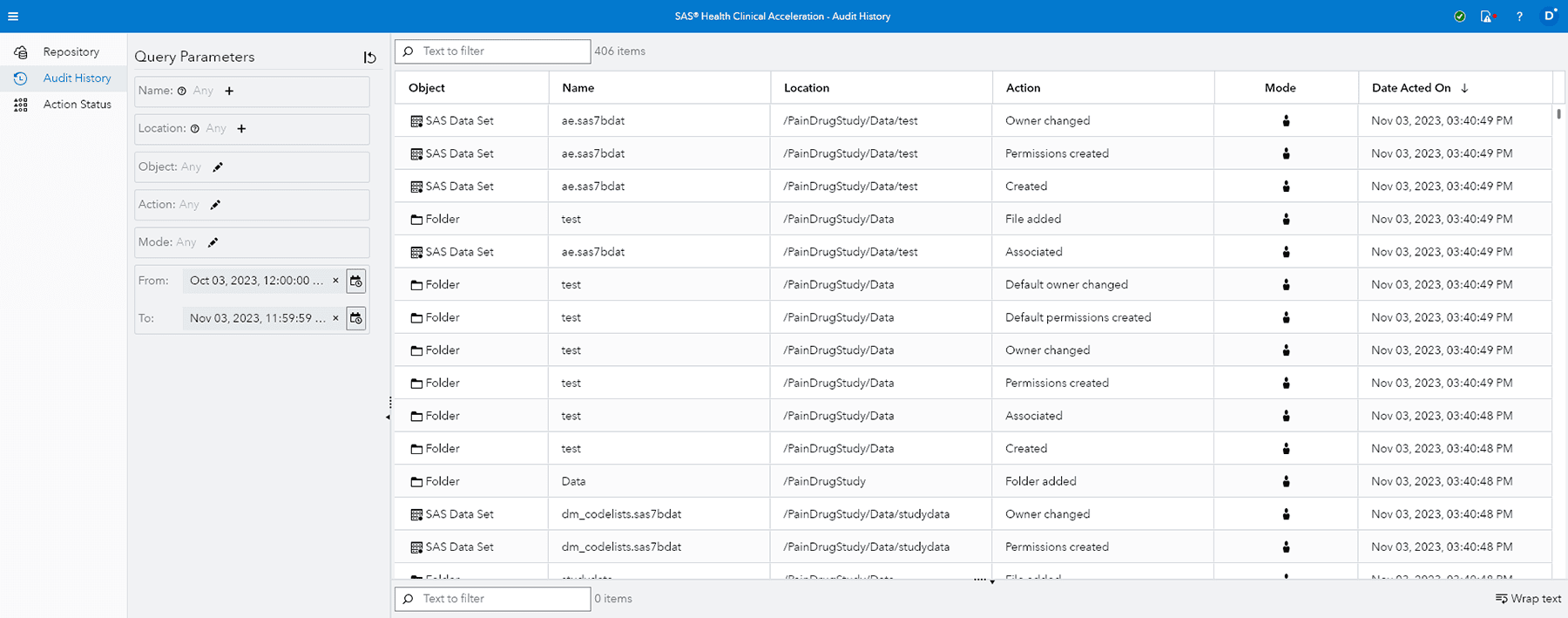
Audit trails
Lets you readily determine what audit changes were made, when and by whom, for all content stored in the repository.
Secure logins
Controls all information and research team access via secure logins.
Regulatory compliance
Enables compliance with the FDA’s Title 21 CFR Part 11 requirements, as well as other industry regulations.
CDISC compliance
Complies with CDISC and its initiatives – dataset-JSON and CDISC CORE.
MDR integration
Supports integration with medical device reporting (MDR).